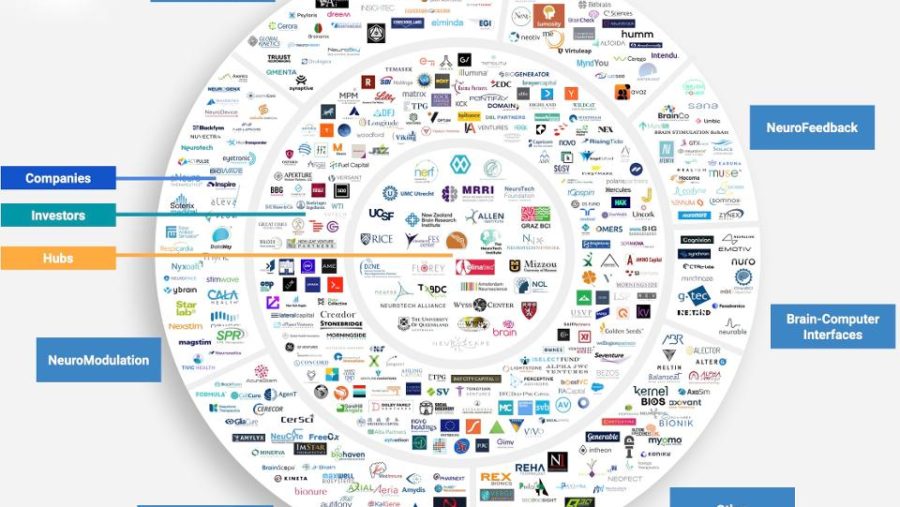
The tES Stimulator designed by us has obtained medical device certificate issued by TÜV NORD – the international certified body.
This document confirms that the technical solutions used in the device are in line with the high requirements of the EU directive on the safety of medical devices. Stimulator software limits the flow of electric current to prevent health hazards. In order to confirm safety, tests were also carried out in specialized laboratory.
We always approach our projects responsibly, bearing in mind that a living person is working with every device. That is why, the issue of device safety is a priority for us.